COVID-19 vaccine: the eight technologies being tested
If the restrictions in place to contain COVID-19 are to end, we need to stop the virus that causes it, SARS-CoV-2, from being able to spread. A vaccine is the most effective and safest way of achieving this, and there is an ongoing global effort to develop one. There are currently around 140 candidates in various stages of development around the world, with a wide range of vaccine types being considered. Some of these have been used for decades to treat human and veterinary diseases, while others are based on techniques that have only recently become available.
The eight main types being tested range from those containing the whole virus, either in a weakened or inactivated form, or those that contain part of the viral structure, to those that depend on our own cells to produce viral proteins that the immune system can recognise. All of them rely on the same basic principle of mimicking a real viral infection and inducing a protective immune response as detailed in the schematic below.
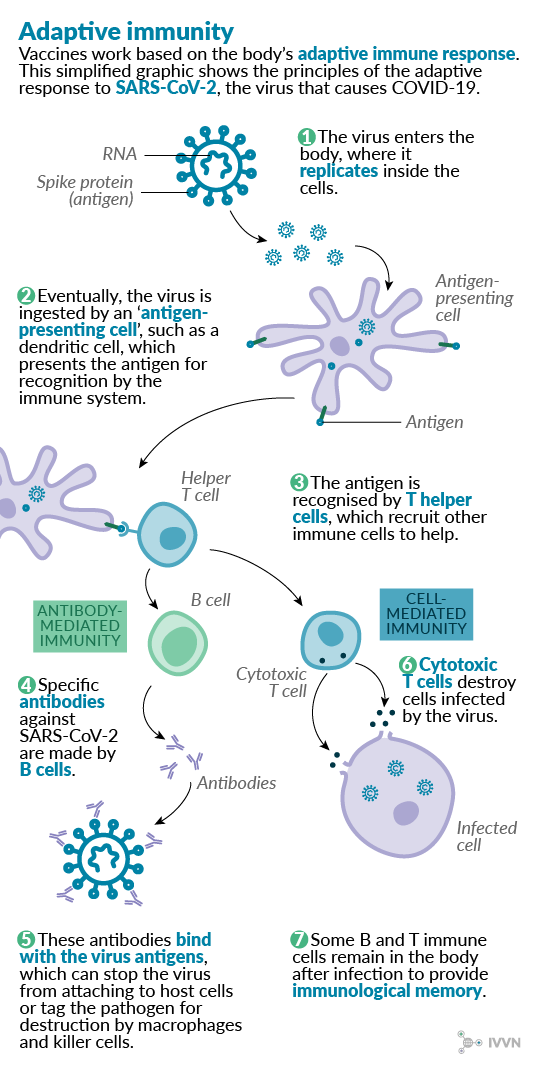
This article explores the advantages and disadvantages of each vaccine type and highlights examples of their use in vaccine strategies for both human and veterinary diseases.
1 Live attenuated virus vaccines
Attenuated virus vaccines contain live virus particles that have been weakened so that they do not cause disease. These vaccines are often cheap to produce and generally give good immunity against infection because they can induce both cell-mediated and antibody-mediated immune responses.1 Many successful veterinary vaccines are live attenuated virus vaccines, including the vaccine used to successfully eradicate rinderpest in the 20th century.2
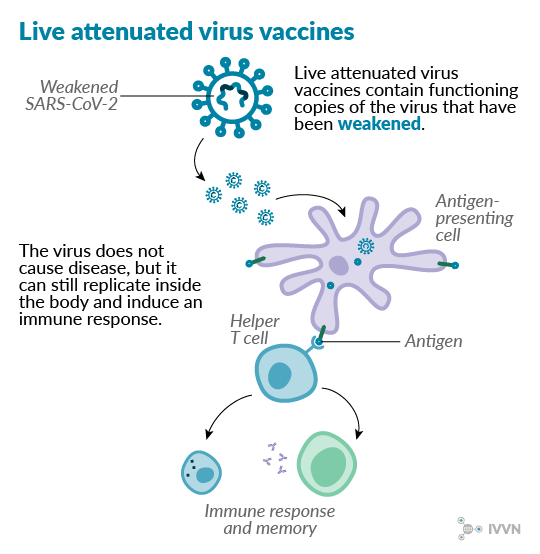
However, there is concern that attenuated viruses in some vaccines could mutate and become virulent. Some live virus vaccines could also be unsuitable for use in people with compromised immune systems, where even a weakened virus could cause disease.
Two attenuated SARS-CoV-2 virus vaccine candidates are in pre-clinical development currently – the first a joint effort by US company Codagenix and the Serum Institute of India, and the second run by Indian Immunologicals Ltd and Australia’s Griffith University.
2 Inactivated (killed) virus vaccines
Similar to attenuated vaccines, inactivated virus vaccines contain whole virus particles, but in this case without live genetic material. The RNA or DNA of the virus is usually destroyed chemically or by heat, leaving the immunogenic elements unaltered. These vaccines elicit an antibody-mediated immune response but usually not a cell-mediated one. This means that, although generally considered safer than live vaccines, the immunity conferred by inactivated vaccines is weaker. Adjuvants – molecules that stimulate the immune system – are often included with inactivated vaccines to give a stronger immune response.
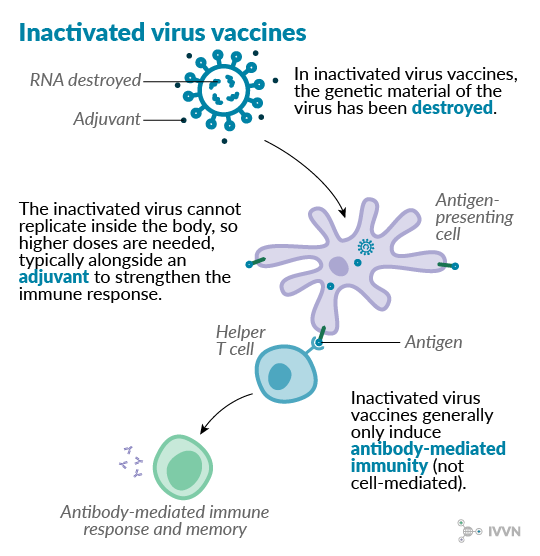
Inactivated vaccines are generally more stable without refrigeration than live vaccines, which has made them popular for veterinary use, especially in low- and middle-income countries (LMICs). The first vaccine against foot-and-mouth disease, developed in 1938 by Waldmann and Köbe, used formaldehyde-inactivated virus particles.3 Many seasonal influenza vaccines used in humans are also inactivated virus vaccines.
Two inactivated SARS-CoV-2 virus vaccine candidates developed by the Wuhan Institute of Biological Products and by Sinovac Biotech are currently in phase I/II clinical trials in China.
3 Viral vector vaccines (replicating)
Replicating viral vector vaccines use a replicating viral vector that has been modified to produce coronavirus proteins in the body. They give a strong immune response and have long been used successfully in poultry, using herpesvirus and poxvirus backbones to immunise against Newcastle disease4 and infectious bursal disease5. In human vaccine development, attenuated measles virus can be used as a replicating vector6, a recent example being a vaccine being developed against chikungunya fever7. One potential disadvantage is that prior immunity to the vector may render the vaccine ineffective in some cases.
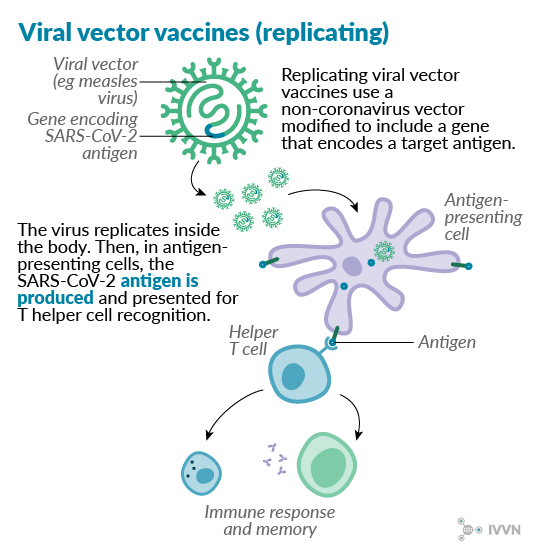
Several SARS-CoV-2 vaccine candidates using replicating vectors are currently at the pre-clinical stage of development, with vesicular stomatitis virus, measles virus and influenza virus among the vectors used. A project at Institut Paseur in France, the University of Pittsburgh in the US and Themis Bioscience in Austria expects to begin testing their measles virus-vectored candidate in animals this month.
4 Viral vector vaccines (non-replicating)
Non-replicating viral vectors – typically adenoviruses – have additional genetic alterations compared with replicating vectors, with key viral replication genes deleted from the vector. Deleting these genes means that larger inserts can be used – up to 8kb in non-replicating adenoviruses, as opposed to 3-4kb without deleting the replication genes6. However, with the vector unable to replicate inside the body, much higher doses are needed to confer immunity. A non-replicating chimpanzee adenovirus vector was recently used to develop a vaccine against Rift Valley fever in small ruminants.8
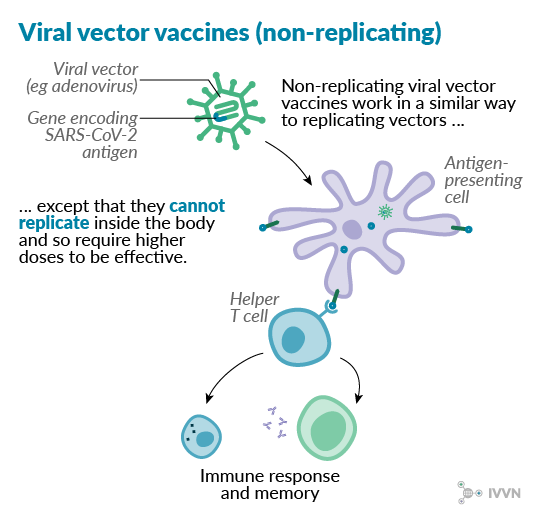
One example of this type of vaccine currently in development against SARS-CoV-2 is the candidate being tested by the Jenner Institute and the Oxford Vaccine Group. This vaccine candidate uses a chimpanzee adenovirus called ChAdOx1 as a vector. Phase I/II clinical trials began in April 2020, with phase II/III trials expected to start later this year.
5 Subunit vaccines
Subunit vaccines are made using the antigenic proteins of the virus without any of the genetic material, which means they cannot replicate inside the body. Because of this, they are considered very safe to use. However, the immune response induced by subunit vaccines is not as strong as for some other types, although this may be improved by using adjuvants. These vaccines may also require several doses to be effective in the long term.
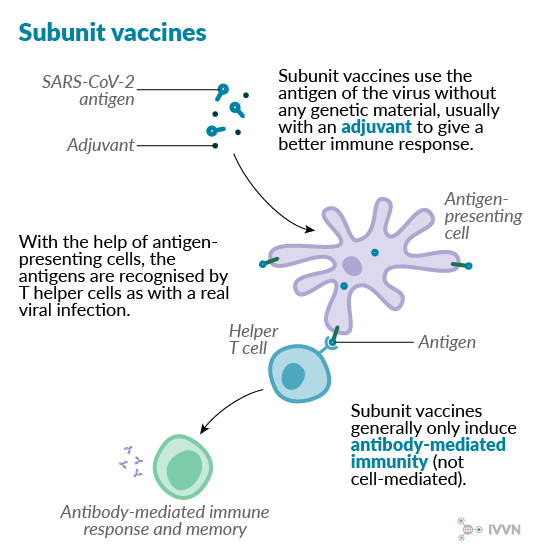
Several subunit vaccines are in use in veterinary medicine, such as many of the vaccines available against porcine circovirus type 2.9 Some subunit preparations are also used to prevent human pathogenic viruses, including some hepatitis B vaccines.
As many as 36 subunit vaccine candidates against SARS-CoV-2 are currently in development. Scientists at VIDO-InterVac at the University of Saskatchewan in Canada are currently testing the safety of their subunit vaccine candidate. The University of Cambridge also expects to begin clinical trials of its subunit vaccine candidate as early as June.
6 Virus-like particle vaccines
Virus-like particles (VLPs) are synthetic protein shells that mimic the structure of a virus, including its antigenic components, without any of the genetic material inside. They have been shown to induce strong immune responses because they preserve the antigen pattern seen on live viruses. They are not infectious and they may be more stable than some subunit vaccines under extreme conditions.10 However, VLPs are more difficult to develop than some other vaccine types and, like subunit vaccines, they may require multiple doses to be effective.
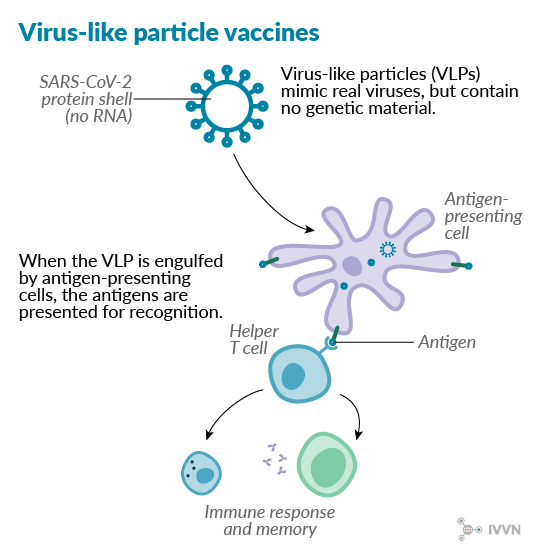
A VLP vaccine against foot-and-mouth disease virus is currently undergoing commercial development, while VLP vaccines already licensed for use in humans include those against human papillomavirus and hepatitis B virus.
There are seven SARS-CoV-2 VLP vaccine candidates being developed, although none have yet commenced clinical trials. ExpreS²ion Biotechnologies in Denmark is currently part of an EU-funded consortium to develop a VLP vaccine candidate using fruit flies to produce recombinant proteins, while Medicago Inc in the US aims to start phase I trials of a plant-produced VLP vaccine by the summer.
7 DNA vaccines
Instead of delivering whole or parts of viruses into the body, in DNA vaccines, a plasmid containing a gene of interest alongside additional genetic elements is delivered into the body, where the antigenic protein is produced. Such vaccines have several potential advantages over conventional types, including production cost and storage requirements. Furthermore, because the antigenic protein is produced inside the body’s cells, a strong cell-mediated immune response is induced as well as an antibody-mediated response.11
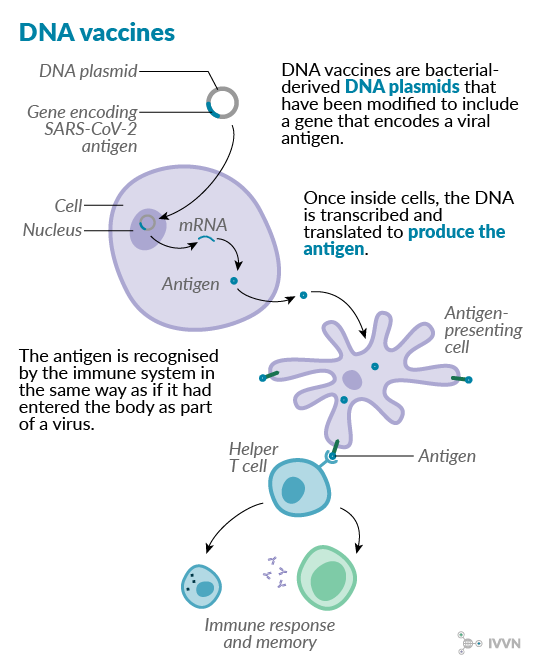
The ease of making DNA vaccines makes them an attractive option for addressing rapidly emerging diseases, but the technology is still relatively new, and no human DNA vaccines are yet licensed. However, several veterinary DNA vaccines have been licensed, including against West Nile virus in horses, and infectious haematopoietic necrosis and pancreas disease infection in salmon aquaculture.
Several SARS-CoV-2 candidates are vying to be the first DNA vaccines used in humans. US-based Inovio Pharmaceuticals, working with four other companies in the US, China and Germany, recently began a phase I clinical trial of its SARS-CoV-2 DNA vaccine candidate. UK-based Scancell, working with scientists at the University of Nottingham, is also testing a DNA vaccine candidate, with development currently in the pre-clinical stage.
8 RNA vaccines
RNA vaccines work by delivering a piece of messenger RNA (mRNA), encased in a lipid coat, into the body where the antigens are produced, in a similar way to DNA vaccines. The antigen-coding mRNA may be accompanied by other mRNA fragments – eg a fragment that will help replicate the RNA inside the cell; this way, fewer copies have to be delivered in the vaccine. RNA is less stable than DNA, and RNA molecules can themselves trigger an immune response in the body, so there has historically been less interest in RNA vaccine development. No RNA vaccines are yet licensed for veterinary or human use. Recent advances, however, have made RNA more stable and less immunogenic, and pre-clinical work on RNA vaccines against Zika and influenza viruses have suggested that they require lower and fewer doses than their DNA counterparts.12
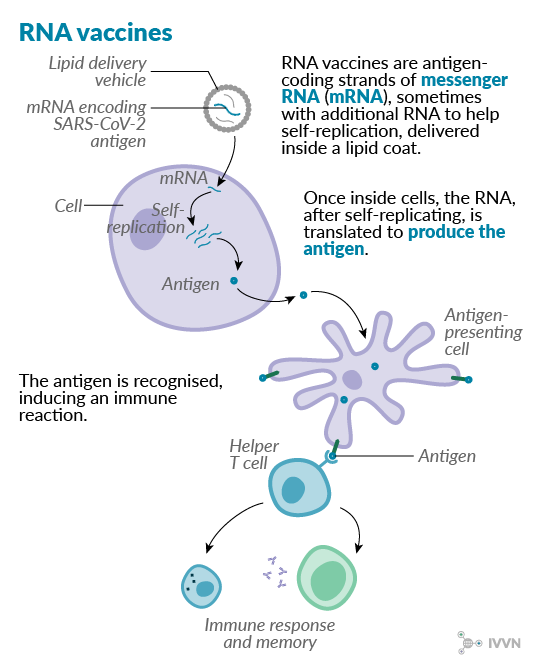
Clinical trials are already under way for two SARS-CoV-2 vaccine candidates – Moderna Therapeutics began its phase I trial in the US in March, while Germany’s BioNTech and US-based Pfizer began their combined phase I/II trial at the end of April. A team at Imperial College London is also among those doing pre-clinical testing of RNA vaccine candidates.
Conclusion
The effort to develop a SARS-CoV-2 vaccine is on a scale we have never seen before. Vaccine researchers around the world have moved at an astonishing pace to help end the pandemic as soon as possible. Whichever projects come up with effective vaccines, the scale and pace of their manufacture are also likely to be groundbreaking. And this massive global effort will have taught us valuable lessons about how we respond to public health emergencies and the importance of vaccines in addressing rapidly emerging diseases.
You can find more information about each of the vaccine candidates being developed and tested worldwide by using the two handy trackers used in writing this article: the Milken Institute’s treatment and vaccine tracker and the London School of Hygiene & Tropical Medicine’s vaccine development pipeline.
References
- van Oirschot (2001) Present and future of veterinary viral vaccinology: a review. Veterinary Quarterly; 23(3): 100-108. doi:10.1080/01652176.2009695094.
- Roeder PL (2011) Rinderpest: the end of cattle plague. Preventive Veterinary Medicine; 102(2): 98-106. doi:10.1016/j.prevetmed.20104.004.
- Lubroth J, Rweyemamu MM, Viljoen G, Diallo A, Dungu B and Amanfu W (2007) Veterinary vaccines and their use in developing countries. Revue scientifique et technique; 26(1): 179-201. doi:10.20506/rst.26.1.1737.
- Palya V, Kiss I, Tatár-Kis T, Mató T, Felföldi B and Gardin Y (2012) Advancement in vaccination against Newcastle disease: recombinant HVT NDV provides high clinical protection and reduces challenge virus shedding with the absence of vaccine reactions. Avian Diseases; 56(2): 282-287. doi:10.1637/9935-091511-Reg.1.
- Rashid MH, Luo H, Akhter J, Islam MT, Islam MR, Rahman MM, Cao Y and Xue C (2014) Protection effect of Vaxxitek HVT + IBD vaccine against infectious bursal disease in broiler chickens. Progressive Agriculture; 24(1-2): 69-78. doi:10.3329/pa.v24i1-2.19102.
- Robert-Guroff M (2007) Replicating and non-replicating viral vectors for vaccine development. Current Opinion in Biotechnology; 18(6): 546-556. doi:10.1016/j.copbio.2007.10.010.
- Zhang L, Gao S and Song S (2019) Recent progress in vaccine development against chikungunya virus. Frontiers in Microbiology; 10:2881. doi:10.3389/fmicb.2019.02881.
- Stedman A, Wright D, Wichgers Schreur PJ, Clark MHA, Hill AVS, Gilbert SC, Francis MJ, van Keulen L, Kortekaas J, Charleston B and Warimwe GM (2019) Safety and efficacy of ChAdOx1 RVF vaccine against Rift Valley fever in pregnant sheep and goats. npj Vaccines; 4: 44. doi:10.1038/s41541-019-0138-0 .
- Chae C (2012) Commercial porcine circovirus type 2 vaccines: efficacy and clinical application. The Veterinary Journal; 194(2): 151-157. doi:10.1016/j.tvjl.2012.06.031.
- Crisci E, Bárcena J and Montoya M (2012) Virus-like particles: the new frontier of vaccines for animal viral infections. Veterinary Immunology and Immunopathology; 148(3-4): 211-25. doi:10.1016/j.vetimm.2012.04.026.
- Jazayeri SD and Poh CL (2019) Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Veterinary Research; 50(1): 78. doi:10.1186/s13567-019-0698-z.
- Pardi N, Hogan MJ, Porter FW and Weissman D (2018) mRNA vaccines—a new era in vaccinology. Nature Reviews Drug Discovery; 17(4): 261. doi:10.1038/nrd.2017.243.