Veterinary vaccines: a silver bullet for animal health in low- and-middle-income countries
Emerging animal diseases remain a major threat to animal and human health as well as to global food security. The burgeoning human population along with increased urbanization and economic growth, primarily in low-and-middle income countries (LMICs), exerts tremendous pressure on existing farm and livestock production systems. Moreover, factors such as unprecedented intensification of animal farming, the globalization of trade in animal/animal products, and climate change are increasing the risk of spreading animal diseases with the potential for huge economic losses.
Important disease prevention and control methods for livestock include farm hygiene, vaccination, biosecurity and zoo sanitary measures. Vaccination is considered an important tool for control of animal diseases outbreaks, especially in endemic areas, and in combination with zoo sanitary measures can offer highly effective disease management.
Historically, vaccination has played an important role and still remains one of the most cost-effective methods of preventing infectious diseases in animals. Veterinary vaccines have played a major role in protecting animal health and reducing animal suffering, thereby enabling efficient production of food animals. Without vaccines helping to reduce animal morbidity and mortality and preventing epizootics among livestock, it would be impossible to meet the demand for animal protein of the nearly 7 billion people on earth. Moreover, without vaccines for companion animals (especially rabies vaccines), many people would not have been able to keep pets and enjoy the benefits of human-animal interactions. Without veterinary vaccines, zoonotic diseases (diseases which can transmit from animals to humans), which constitute around 61% of the total number of infectious diseases and 75% of the total emerging diseases, would have been much more prevalent and a much greater threat to public health. Veterinary vaccines for important viral zoonoses (Rabies, Rift Valley Fever, Japanese encephalitis etc.) and bacterial zoonoses (Anthrax, Tuberculosis, Brucellosis, Leptospirosis, Q fever etc.) have been, or could be, used to prevent and control infections in animals, therefore reducing the probability of transmission to humans. Wildlife vaccines (e.g. oral bait vaccines for rabies, Brucellosis vaccine for bison and elk etc.) help in the prevention of spill-over of infectious agents to domestic animals and, in cases of zoonoses, to humans. In addition, vaccines are one of the available ‘silver bullets’ that have the capacity to reduce the need for antibiotics to treat food and companion animals and thereby contribute to combating the problem of antibiotic resistance.
Historical and recent approaches in vaccine development
Historically, most of the vaccines have been of two types: live attenuated vaccines and inactivated vaccines. The live attenuated vaccines are capable of providing long-lasting immunity but with a compromise between virulence, immunogenicity and safety. Many of these vaccines have inherent shortcomings, such as residual infectivity, interference with maternal immunity, reversion to virulence, and problems with thermostability. The reversion to virulence is perhaps the greatest risk associated with live attenuated vaccines, potentially causing not only disease in vaccinated individuals but also possible shedding of pathogens into the environment, enhancing the risk of transmission to others. There also remain risks of reassortment and recombination between live attenuated vaccine strains and virulent strains circulating in the field. The thermostability of many of these vaccines has also been a point of great concern considering that the vaccines have to be transported over long distances to remote areas where cold chain maintenance is difficult in many LMICs.
Inactivated vaccines are generally non-infectious but often regarded as inferior in stimulating immunity in comparison to live attenuated vaccines. They lack the ability to directly activate cell-mediated immune responses, therefore the efficacious response of these vaccines is entirely dependent on the adjuvant used. Veterinary vaccines use a wide variety of novel adjuvants that are not yet approved for use in human vaccine production. The choice of adjuvant used may have effects on vaccine parameters, for example, oil-adjuvant vaccines (e.g. vaccine for hemorrhagic septicemia), though known to be potent, are difficult to inject in to animals on account of their high viscosity.
From the 1980s onwards, the biotechnological and associated proteomic advances led to the development of so-called ‘second-generation vaccines’. There are many successful examples of veterinary vaccines which have been generated using novel technologies that are licensed for use including: gene-deleted marker vaccines, recombinant modified live virus vaccines, subunit vaccines, vectored vaccines, DNA vaccines, synthetic protein vaccines, edible vaccines and other chimeric vaccines. Recombinant technology assisted by high performance bioinformatics has made the identification of potential markers for vaccines that comply with the DIVA (differentiation of infected and vaccinated animals) principle much more feasible and so provide a much-needed tool for using vaccines in field situations where discriminating vaccinated (and protected) from non-vaccinated (and potentially disease transmitting) animals is critical. The technological advancement in recombinant live vaccines generates the possibility of producing multivalent vaccines, which can help in addressing more than one disease at a given time. The possibility even exists that multiple protective epitopes from a variety of pathogens could be concatenated to create a single protein antigen that could be deployed in vaccines. Such innovations are particularly important for LMICs where reducing the cost of vaccination campaigns can be pivotal in the feasibility of governments funding larger interventions. Recombinant subunit vaccines also eliminate the risks associated with handling vaccines that contain live inactivated pathogenic organisms. Recent advances have also made the rapid identification of protective epitopes, including cross-species identification of functionally similar proteins, possible and with it the potential to both enhance the efficiency in the development of novel vaccines and also the efficacy of such subunit vaccines. Together the combination of recombinant technology, bioinformatics and genomics has revolutionized approaches to vaccine candidate discovery including ‘reverse vaccinology’ approaches that start with pathogen’s genome as the source material to begin antigen identification (which can therefore begin even before the emergence of the pathogen as a cause of a disease outbreak).
A major constraint on the use of novel technologies for veterinary vaccine production is the potential financial returns, which may be much lower than from human vaccines - mainly due to lower sales prices and smaller potential markets. As a consequence, it is more difficult for industry to be able to justify the level of investment in R&D for veterinary vaccines on a par with that of human vaccines. It is therefore critical for academic, industrial and other stakeholders to pro-actively seek cross-sector engagement. Additionally, the community as a whole must ensure that investments made in veterinary vaccine research are optimized to have the best chance of generating products that are suitable for commercialization and can meet the necessary safety, efficacy and regulatory standards without increasing the cost of production and licensing to the point where they are not affordable to the end user.
Factors affecting vaccination strategy
Decisions on timing, location and extent of immunization campaigns should be carefully considered with the design of a vaccination strategy taking into account important economic and sociological factors. Vaccination may be applied in a variety of ways including as routine prophylactic or emergency vaccination. Some of the factors which may affect the choice of vaccination strategy are listed below:
- Host, pathogen and disease progression characteristics
- Parameters associated with economic aspects of disease and their control measures
- Nature of the outbreak (live vaccines cannot be used during an outbreak)
- Livestock farm characteristics (extensive/semi-intensive/intensive system)
- Existence and availability of appropriate vaccines(s) in terms of efficacy
- Farmers' perceptions about disease risk and economic consequences
- Availability of human resources (trained personnel) and financial capacity
- Implementation of co-measures such as, biosecurity and zoosanitary measures
Concept of herd immunity and vaccine coverage
Herd immunity can be defined as ‘indirect protection from infectious disease that occurs when a large percentage of a population has become immune to an infection, thereby providing a measure of protection for individuals who are not immune’. Herd immunity is an important element in the balance between the host population and the pathogen, and represents the degree to which the host population is susceptible (or not) to an infectious disease spreading and being maintained in the population as a consequence of animals of that population having acquired active immunity from either previous infection or prophylactic immunization.
The decision whether to introduce herd immunity by immunization against a particular disease will depend on several epidemiological parameters which includes vaccine efficacy (Ve), vaccine coverage (Vc) and the basic reproduction number of the pathogen (R0). R0 defines the number of new infection(s) each infected case can cause (so highly infectious pathogens have a high R0). To decrease the spread of pathogens it is necessary to use vaccines to achieve a level of protected animals (Pc) that exceeds the herd immunity threshold (HIT) which is defined as:
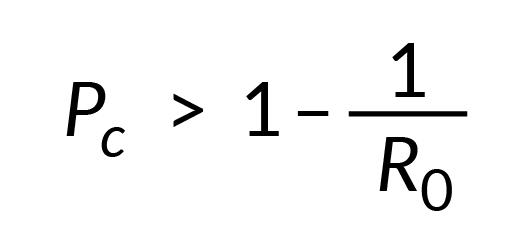
As the proportion of animals in a herd that will be protected by vaccination will be determined both by vaccine coverage (Vc) and vaccine efficacy (Ve) then Pc from vaccination can be calculated as = Vc x Ve and consequently the vaccine coverage required to achieve effective herd immunity as:
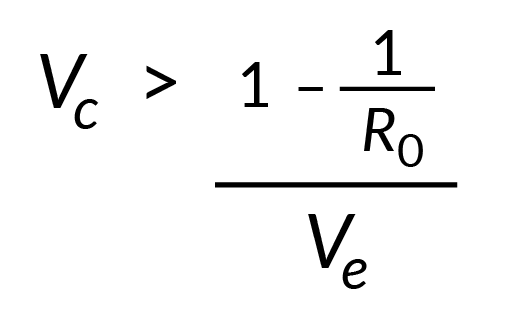
Vaccine related challenges in LMICs
Infectious diseases can cause huge livestock mortality and other production losses which can make the farming community susceptible to malnutrition and many zoonotic diseases in LMICs. Before implementing any prevention and control strategy for infectious diseases, initial impact assessment and decision analysis is necessary in order to assess the relative merits of different disease intervention options, including vaccination. Novel multivalent vaccines offer a more effective and labour saving approach to generate protection against a range of circulating strains or variants of disease but there is a significant cost to such a strategy which affects their economic affordability in LMICs. Many of the disease control programs that utilize vaccination solely on the basis of voluntary uptake are at risk of failure if willingness to vaccinate is too low to reach satisfactory vaccination coverage (Vc) and thus generate the level of herd immunity required to control the spread of the disease.
In many places, the lack of cold chain maintenance facilities remains a major constraint to get the desirable results of vaccination programs. Moreover, in some places the substandard biological testing procedures during the vaccine production chain may lead to production of vaccine with inappropriate efficacy. Therefore, proper biological standards and production controls in the manufacture of veterinary vaccines and sufficient infra-structure for vaccine distribution are essential for ensuring quality products and so vaccine efficacy (Ve).
On the economic front, if veterinary vaccines are to be effective in contributing to animal health, they must be affordable so that their use is sufficiently widespread and sustained. In LMICs, the farmer (who bears the cost of vaccination and other veterinary services) must be aware of the animal health economic benefits of vaccination and convinced that this is a valid use of limited financial resources. The availability of competent veterinary infrastructure and the wider social and political perception about use of vaccines are critical to the success of vaccination campaigns in LMICs. The anti-vaccination campaign, which continues in both high-income countries (HICs) and LMICs, is posing a major hindrance to successful vaccine coverage programs. Since prophylactic vaccination does not cause any immediate obvious benefit to immunized animals, education of farmers to the benefits of vaccination is critical. Equally in scenarios where the prevalence of infectious diseases among animals is high, effective vaccination strategies are dependent on strong surveillance systems and the deployment, where available, of DIVA (differentiation of infected and vaccinated animals) vaccines.
Conclusion
Although much progress has been made in vaccine development techniques in recent decades, many significant challenges remain unmet. Effective partnerships between vaccine manufacturers, funders, stakeholders and policy-makers will be necessary to resolve these challenges. The regulatory process for evaluation of vaccines must ensure adequate evaluation of biologicals in order to meet the basic criteria such as purity, safety, potency and efficacy. Veterinary vaccines must also be economical to encourage their widespread prophylactic use and thereby decrease disease incidence and so farmer dependency on other therapies such as antibiotics, especially critical in the current situation of escalating anti-microbial resistance (AMR). Finally, vaccination approaches must be complemented with other disease prevention and control strategies that include detailed understanding of the disease ecology and epidemiology, biosecurity measures, robust surveillance system, proper diagnostic procedures, education and awareness, and control of the disease vector and/or reservoir species.